Abstract
Background: Patients with newly diagnosed multiple myeloma (NDMM) undergoing upfront autologous stem cell transplantation (ASCT) have different characteristics worldwide, likely due to variances in transplant activity, patient factors and health economic factors including access to new myeloma therapies. The first aim of this retrospective study was to analyze the global baseline patient characteristics and outcomes of upfront ASCT in NDMM.
Methods: Data on patient characteristics and transplant outcomes for patients with NDMM who received an upfront ASCT between 2013 and 2017 were provided to the Worldwide Network for Blood and Marrow Transplantation (WBMT) by the European Society for Blood and Marrow Transplantation (EBMT), the Center for International Blood and Marrow Transplantation (CIBMTR), the Asian Pacific Blood and Marrow Transplant Group (APBMT), the Australia and New Zealand Transplant and Cellular Therapies Registry (ANZTCT), the Eastern Mediterranean Blood and Marrow Transplant Group (EMBMT), the Latin American Bone Marrow Transplant Group (LABMT), and the Ottawa Canadian Registry. There primary endpoints were overall (OS) and non-relapse mortality (NRM) and the secondary were progression-free survival (PFS) and relapse incidence (RI). The Kaplan-Meier estimator and log-rank test were used for OS and PFS, and the crude cumulative incidence estimator and Gray's test were used for competing events (RI and NRM). Age at ASCT, sex, year of ASCT, disease stage, Karnofsky score, MM classification, preparative regimen, time between diagnosis and ASCT, ISS stage at diagnosis, cytogenetic risk, and HCT-CI score were studied for prognostic value. Multivariable (cause specific) models included a random effect for each country and were censored at 3 years for OS and PFS and at 1 year for RI and NRM.
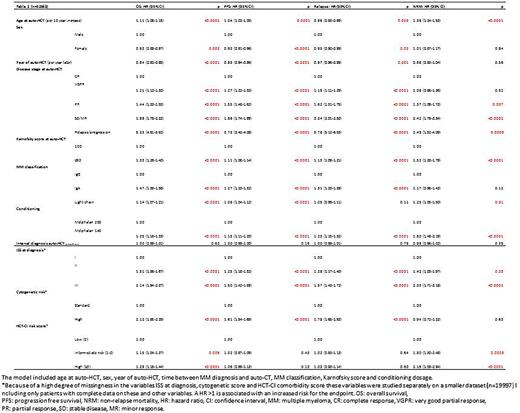
Results: 61,725 patients from 629 centers (median patients/center = 67) were included: 61% from EBMT, 26% CIBMTR, 6% APBMT (5% Japan, 1% Taiwan, 0.3% Malaysia), 5% AZTCT, 1% EMBMT, 0.5% LABMT and 0.3% Ottawa, Canada. The patient baseline characteristics are shown in Table 1. Males comprised 58% and the median age at diagnosis was 60 years. The predominant phenotypes were IgG (54%), light chain (24%) and IgA (19%). The ISS stage at diagnosis was I (38%), II (35%) or III (27%) and cytogenetic risk was standard in 70% and high in 30%. The median time from diagnosis to ASCT was 7.1 months. The year of ASCT was equally distributed between 2013 and 2017 (the annual percentage varied between 18% and 22%). The median age at ASCT was 60.8 years with 5.1% of patients older than 70 years. The HCT-CI risk at transplant was low in 52%, intermediate in 25% and high in 23%. The Karnofsky score at ASCT was 100 in 40% and ≤90 in 60%.Disease status was CR in 19%, VGPR in 38%, PR in 36%, MR/SD in 5% and refractory in 2%. The most frequent preparative regimen was melphalan 200 mg/m2 (82 %) and 140 mg/m2 comprised 14%. Tandem ASCT was reported in 6.7%. Of the 11% of patients with data on post-ASCT maintenance treatment, 51% received lenalidomide. The median follow-up was 41.1 months (95% CI: 40.5 to 41.6, IQR:19.2-60.4). Outcomes: non-relapse mortality (NRM) at 12 months was 1% (95% CI 1-2%); OS at 4 and 8 years was 76% (75-76%) and 45% (42-48%) respectively; PFS at 2 and 4 years was 65% (95% CI: 64-65%) and 40% (40-41%) respectively; RI at 6 and 12 months was 7% (7-7%) and 16% (15-16%) respectively. In the multivariate analysis (Table 2), later calendar year of ASCT, a better disease response at time of ASCT, higher Karnofsky score, and an IgG phenotype were all associated with an improved OS. A lower ISS stage, a lower HCT-CI score and standard risk cytogenetics were also associated with better OS (Table 2). Overall NRM was low. Younger age, higher Karnofsky score, higher melphalan dose, better disease response at ASCT, lower ISS stage at diagnosis and lower HCT-CI score were associated with a lower NRM.
Conclusions: This study represents the largest study to date characterizing the outcomes of ASCT performed worldwide. The most frequent preparative regimen was melphalan 200 mg/m2. Unmeasured confounding (pre-existing toxicities/frailties) may partially explain the higher NRM in those with low dose melphalan. Globally, NRM was low at 1% at 12 months, while RI was 16%. Median OS was 7 years, median PFS was 3 years and differed across various risk factors. ASCT remains an effective and safe procedure for patients with NDMM world-wide.
Disclosures
Garderet:Sanofi: Honoraria; BMS: Honoraria; Janssen: Honoraria. Hari:Amgen: Consultancy, Honoraria; Pharmacyclics: Consultancy; AbbVie: Honoraria; GlaxoSmithKline: Honoraria; Janssen: Consultancy, Honoraria; Novartis: Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Incyte: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria; Iovance: Current Employment; Millennium: Research Funding; Spectrum Pharmaceuticals: Research Funding. Cowan:Abbvie: Consultancy, Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Allogene: Consultancy; BMS: Consultancy, Research Funding; EUSA: Consultancy; GSK: Consultancy; Harpoon: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Research Funding; Sanofi: Research Funding; Secura Bio: Consultancy. Takamatsu:Bristol-Myers Squibb: Honoraria; Sanofi: Honoraria; Ono: Honoraria; Janssen: Honoraria; SRL: Consultancy. Hamad:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Mian:Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria. McCurdy:Takeda: Honoraria; BMS: Honoraria, Research Funding; Janssen: Honoraria; GSK: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Forus: Honoraria. Snowden:Medac: Membership on an entity's Board of Directors or advisory committees; Janssen and Jazz: Speakers Bureau; Mallinckrodt: Speakers Bureau; Gilead: Speakers Bureau; Kiadis: Other: clinical trial IDMC membership ; Novartis: Speakers Bureau. Schönland:Pfizer: Honoraria; Takeda: Honoraria, Other: Travel Support; Janssen: Honoraria, Other: travel support, Research Funding; Prothena: Honoraria, Other: Travel Support, Research Funding. McLornan:NOVARTIS: Honoraria, Research Funding, Speakers Bureau; JAZZ: Honoraria, Speakers Bureau; ABBVIE: Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau. Hayden:Amgen: Other: Participation in Advisory Board. Sureda:SANOFI: Consultancy, Honoraria; BMS: Consultancy, Honoraria; GILEAD: Consultancy; MSD: Honoraria; JANSSEN: Consultancy, Honoraria; ROCHE: Consultancy, Honoraria; NOVARTIS: Consultancy, Honoraria; TAKEDA: Consultancy, Honoraria, Speakers Bureau. Greinix:Gilead, Novartis, Sanofi, Cellgene: Consultancy; Amgen, Gilead, Novartis, Sanofi, Takeda, Therakos: Speakers Bureau. Atsuta:Novartis Pharma KK: Honoraria; Astellas Pharma Inc.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria; AbbVie GK: Honoraria; Mochida Pharmaceutical Co., Ltd.: Honoraria; Meiji Seika Pharma Co, Ltd.: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal